Unknown Project: Fungal Genera I and II
Purpose
The purpose of this project is to isolate and identify genera of fungi not listed in Group I of a picture key of common molds available at the following website: http://website.nbm-mnb.ca/mycologywebpages/Moulds/ID_Plate_I.html.
Materials and Methods
15% water agar medium amended with chloramphenicol, mefenoxim and propiconazole
20% water agar medium
Potato Dextrose Agar medium
Flat-sided, wooden toothpicks
Tweezers
Scalpel
Needle-nosed pliers
Red and black permanent markers
Orange marking paint
70% ethanol
50-ml beaker
Bunsen burner
Metal striker
Paper towels or bench cover
Parafilm
Incubator set at 25 degrees Celsius
Compound microscope
Canon PowerShot SD550 digital camera
A. Fungal isolation and culture conditions
Orange spray paint was used to mark several ~5 cm spots in 'Palisades' zoysiagrass at an outdoor Texas A&M Turfgrass Research Laboratory on F&B Road in College Station, Texas, on September 8, 2012. Four flat-sided wooden toothpicks (Jarden Home Brands, Daleville, IN), which had been colored on the rounded end (~6 mm) with a red permanent marker, were then inserted into the turfgrass in a cross pattern, just outside each spot and just up to the bottom of the red mark (Figure 1).
Orange spray paint was used to mark several ~5 cm spots in 'Palisades' zoysiagrass at an outdoor Texas A&M Turfgrass Research Laboratory on F&B Road in College Station, Texas, on September 8, 2012. Four flat-sided wooden toothpicks (Jarden Home Brands, Daleville, IN), which had been colored on the rounded end (~6 mm) with a red permanent marker, were then inserted into the turfgrass in a cross pattern, just outside each spot and just up to the bottom of the red mark (Figure 1).
 |
| Figure 1. Configuration of toothpicks inserted into turfgrass to bait for fungi in soil and thatch. |
Toothpicks were removed after two days using needle-nosed pliers, placed into snack-sized zip-lock plastic bags labeled to correspond to the baited spot, and then taken to a laboratory in the L.F. Peterson Building on the Texas A&M University campus in College Station. Using a sterile technique described in my blog for Lab 2, alcohol and flame-sterilized tweezers were used to remove toothpicks from bags and plate them onto 15% water agar (WA) media that was prepared on September 9, 2012, and had been amended with chloramphenicol, mefenoxam 2AQ (Quali-Pro, Pasadena, TX) and propiconazole (Quali-Pro, Pasadena, TX) (Figure 2).
 |
| Figure 2. Configuration of toothpicks on isolation medium. |
Plates were incubated at 25 degree Celsius in the dark for approximately 18 hours, and then toothpicks were examined from the underside of plates with a compound microscope at 4X and 10X magnification. Location of hyphal tips of fungi growing from the sides of toothpicks were marked on plates with a black permanent marker. Using the previously described sterile technique, hyphal tips were transferred with an alcohol and flame-sterilized scalpel to 20% WA media. The plates were sealed with parafilm, and incubated and observed daily as previously described. Hyphal tips that grew out of agar plugs were transferred to new 20% WA plates after 2-3 days to ensure purity of cultures. The plates were sealed with parafilm, and incubated and observed daily as previously described. Plugs from the edges of pure fungal colonies were transferred to Potato Dextrose Agar (PDA) media to facilitate production of sexual or asexual structures to aid in identifying the fungi in addition to culture morphology. The plates were sealed with parafilm, and incubated and observed daily as previously described. A second passage on PDA was performed on September 24, 2012, to again ensure purity of cultures. Plates of pure cultures were photographed on September 28, 2012.
B. Identification of fungus to genus
Fungi isolated from zoysiagrass using the toothpick baiting method were identified based on culture morphology and production of asexual structures (conidia). I used a published reference (Figure 3) to confirm the identities of my isolations.
Results
 |
| Figure 3 |
A. Fungal genera I
+9-28-2012.jpg) |
| Curvularia sp. on PDA (top of culture). |
+9-28-2012.jpg) |
| Curvularia sp. on PDA (bottom of culture). |
 |
| Brown, septate hyphae of Curvularia sp. ~2 days after subculturing from the isolation medium onto 20% WA (4X). Note spiraling of hyphae. Photograph cropped and enlarged to show detail. |
+9-28-2012.jpg) |
| Bipolaris sp. on PDA (top of culture). |
+9-28-2012.jpg) |
| Bipolaris sp. on PDA (bottom of culture). |
Discussion
Detection of fungi from soil can sometimes be difficult. Thus, as part of my doctoral research to detect and monitor the soil-borne fungus Rhizoctonia solani that causes large patch in warm-season turfgrasses, I have developed a baiting method using flat-sided wooden toothpicks. The method is a further modification of one first developed by Kumar et al. (1999) (Canadian Journal of Microbiology 45:389-395) to measure the linear
spread of R. solani AG-8 and AG-11 in sand in greenhouse assays. Paulitz and Schroeder (2005) (Plant Disease 89:767-0772) modified the method to facilitate both isolation and quantification of R. solani
AG-8 and R. oryzae inside and outside
of bare patches in wheat fields in eastern Washington. It is known that R. solani can decompose cellulose (Garrett. 1962. Transactions of the British Mycological Society 45:115-120). Paulitz and Schroeder (2005) surmised that the interaction between Rhizoctonia sp. and a
toothpick is thigmotrophic rather than chemotrophic, and expected hyphae to attach
and grow along the toothpick surface.
During the past year, I have successfully isolated Rhizoctonia sp. from soil and thatch in zoysiagrass using wooden toothpicks as bait. I also have had no problem isolating Fusarium, Phytophthora, Pythium and unknown bacterial species. None of these can be used for my unknowns project in PLPA 631, as the first is among Group I of a picture key of common molds, the next two are Oomycetes and not Fungi, and the last - well, this is a fungal laboratory course.
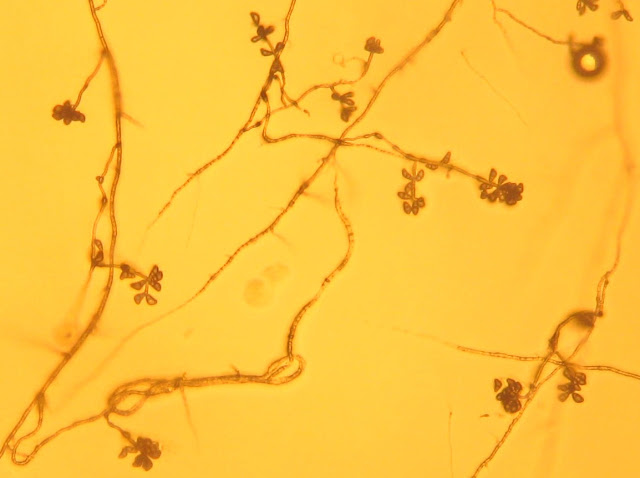







Do you have other Bipolaris images that are more clear. I'm not sure what the conidia look like based on that image.
ReplyDelete