Fundamentals of microscopic
observation of fungal structures
observation of fungal structures
Purpose
The objectives of this laboratory exercise on September 5, 2012, were to 1) learn sterile technique in preparing microscope slides from pure cultures at a laboratory work bench 2) learn to prepare tape and squash mounts to microscopically observe fungal structures 3) learn to set Köhler on a compound microscope to produce illumination of specimens that is uniformly bright and free from glare 4) learn to count fungal spores in a suspension using a hemocytometer.
Materials and Methods
Fungal cultures of Thielaviopsis basicola, Cladosporium sp., Alternaria brassicicola, Aspergillus niger and Pythium ultimum
Microscope slides
Cover slips
Clear tape
Microcentrifuge tubes
Scalpel
Dissecting needle
20 ul pipettor with tips
Dropper bottle with water
Spray bottle with 70% ethanol
Paper towels
Metal striker
Bunsen burner
Hemocytometer
Olympus CX31 compound microscope
A. Sterile technique in preparing microscope slides
Materials and Methods
Fungal cultures of Thielaviopsis basicola, Cladosporium sp., Alternaria brassicicola, Aspergillus niger and Pythium ultimum
Microscope slides
Cover slips
Clear tape
Microcentrifuge tubes
Scalpel
Dissecting needle
20 ul pipettor with tips
Dropper bottle with water
Spray bottle with 70% ethanol
Paper towels
Metal striker
Bunsen burner
Hemocytometer
Olympus CX31 compound microscope
A. Sterile technique in preparing microscope slides
- A paper towel was laid on the work bench, and supplies needed to prepare a fungal specimen for microscopic observation (slide, cover slip, scalpel, dropper bottle with water) were assembled on the towel along with a pure fungal culture.
- A Bunsen burner was located next to the paper towel, the gas jet opened, and the burner ignited with a metal striker. The height of the flame was adjusted to about 4 inches.
- The blade of the scalpel was sterilized by passing it through the flame of the Bunsen burner and then was cooled by touching it to non-colonized medium at the outer edge of the culture.
- An approximately 2 mm-square plug was excised from the outer edge of the fungal colony with the flame-sterilized scalpel and placed on the center of a microscope slide. A drop of water and then a cover slip were then applied to the top of the fungal plug. The handle of the scalpel was used to gently push down on the cover slip to squash the fungal plug.
Dr. Brian Shaw gave instructions on preparing squash and tape mounts to microscopically observe fungal structures. Squash mounts were prepared from fungal cultures as previously described in Step 4 of Part A of the Material and Methods section in this report. To prepare a tape mount, the sticky side of an approximately 1-inch-long piece of clear tape was lightly touched to the surface of a fungal culture and then affixed to a microscope slide on which a drop of water had been placed.
C. Setting Köhler on a microscope
Dr. Brian Shaw gave instructions on setting Köhler on a compound microscope. To set Köhler, I prepared a squash mount from a pure culture of T. basicola and placed the slide on the stage of an Olympus CX31 compound microscope. The specimen was brought into focus using the 4X objective. The annulus located in the base of the microscope was then closed by turning a black ring. A fuzzy octagon-shaped point of light, representing the iris, was visible through the eyepiece and was centered in the field of view using two silver knobs below the microscope stage. The edges of the octagon were then brought into focus, and a red-blue cast eliminated, by turning a black lens knob on the side of the condenser. Finally, the annulus was reopened by turning the black ring in the base of the microscope until light filled the field of view through the eyepiece.
D. Counting fungal spores with a hemocytometer
- Adhering to sterile technique previously described in Part A of the Materials and Methods section in this report, a dissecting needle was used to lightly scrape the outer edge of a fungal colony.
- The tip of the needle then was twizzled into 1 ml of water in a 1.5-ml microcentrifuge tube, and the tube gently flicked to distribute spores.
- Approximately 10 ul of the spore suspension was pipetted into each of the two chambers of a hemocytometer (Figure 1), and spores were observed microscopically and counted (Figures 2 and 3), according to instructions on a YouTube video http://www.youtube.com/watch?v=pP0xERLUhyc.
Figure 1. A hemocytometer with two chambers or wells for loading fungal spores in suspension. http://static.coleparmer.com/large_images/3621900.jpg
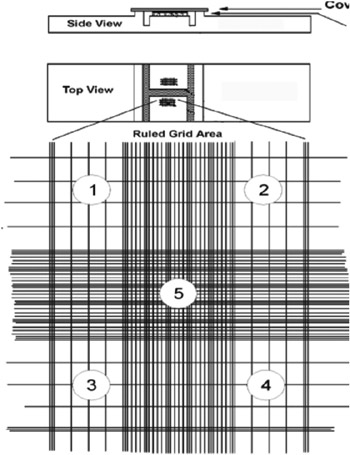
Figure 2. Ruled grid area in a hemocytometer for counting spores.
http://www.globalspec.com/reference/54403/203279/EXERCISE-6-CELL-COUNT-BY-HEMOCYTOMETER-OR-MEASURING-VOLUME
http://www.globalspec.com/reference/54403/203279/EXERCISE-6-CELL-COUNT-BY-HEMOCYTOMETER-OR-MEASURING-VOLUME

Figure 3. One of four corner squares in a hemocytometer grid used to count and calculate the number of fungal spores in a suspension.
http://amrita.vlab.co.in/?sub=3&brch=188&sim=336&cnt=2
http://amrita.vlab.co.in/?sub=3&brch=188&sim=336&cnt=2
Results
A. Microscopic observation of fungal structures
B. Determining number of fungal spores in suspension
Spores of A. niger were suspended and counted using a hemocytometer. The number of viable cells was not determined using trypan blue stain.
Average number of spores per square: (250/5) = 50
Dilution factor: (1000/1000) = 1
Concentration of spores: 50 x 1 x 10e4 = 500,000 or 5 x 10e5 spores per ml
Discussion
Tape mounts were preferred to squash mounts for observing more delicate fungal structures such as those produced by P. ultimum. All five fungal cultures produced an abundance of spores, especially A. niger. One slight scrape with a dissecting needle was more than adequate to pick up enough spores for counting with a hemocytometer.
 l
l

No comments:
Post a Comment